
To find the electron configuration of other elements of the periodic table you need to click on recalculate button.įAQ: What are the main rules for electron configuration?.This best ground state electron configuration calculato r provides abbreviated electron configuration, standard state, atomic mass, and the number of an element.Hit to calculate button to get electron configuration mnemonics.Enter an element to find the complete electron configuration of an element.
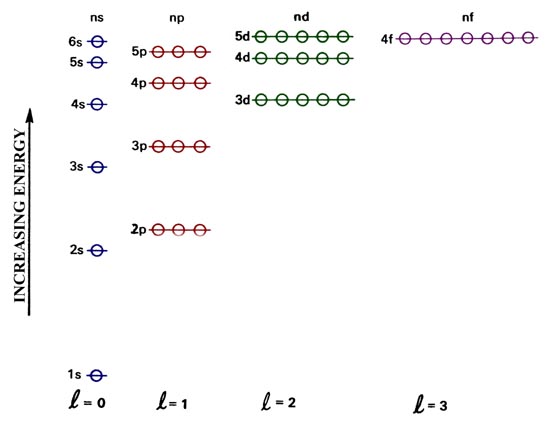
How Valence Electron Configuration Calculator Works?Īn online valence electrons calculator finds the abbreviated or condensed electron configuration of an element with these instructions: Input: However, an Online Photon Energy Calculator will allow you to find the energy of a photon from its wavelength & frequency. The atomic mass unit (AMU) is usually used to measure atomic mass.Īn atomic number is a number that is used to place elements in the periodic table. It is the total number of nucleons in the atom’s nucleus. It is the average weight of an atom or molecule. The atomic number is usually the number of protons present in the nucleus of an element that you can also determine using this best atomic number calculator. Atomic MassĪtomic mass is related to the number of neutrons and protons present in a particular nucleus of an element. It is believed that the atomic mass of an element is closely related to the atomic number, because if the atomic mass is high, then the atomic number is also high, what is the difference between atomic mass and atomic number? To find out, let’s look at some key differences. Difference Between Atomic Number and Atomic Mass: The values of ℓ = 0, 1, 2, and 3 correspond to the orbit s, p, d, and f, respectively. An atom with an nth electron shell can hold 2n^2 electrons, which is the first shell that can hold 2 electrons, the second shell can hold 8 electrons, and so on.Ī subshell is a set of states, which are defined by the total azimuth quantum number “l” in the shell. Shells and Subshells:Įlectron shells are a set of feasible states that have the same principal quantum number n (the number before the letter on the orbital) that the electron can occupy. However, an Online Angular Velocity Calculator allows you to determine the angular velocity of the body in motion on a circular path.
#Krypton orbital diagram free#
#Krypton orbital diagram series#
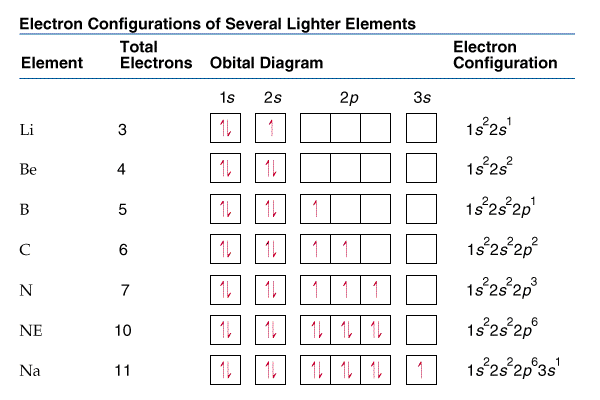
For atoms, the standard notation consists of a series of atomic subshell labels (for example, phosphorus sequence of notation is 1s, 2s, 2p, 3s, 3p), where the number of electrons assigned to each subshell is used as a superscript.
#Krypton orbital diagram how to#
Usually, Physicists and chemists use the isotope notation calculator to refer how to calculate electronic configurations of molecules and atoms. For example, electron configuration of Phosphorus (P) is 1s^2 2s^2 2p^6 3s^2 3p^3. It also describes every electron as moving freely in an orbital, in an average field generated by other orbitals. In quantum chemistry and atomic physics, the electron configuration of an atom or molecule describes the distribution of electron distribution mnemonics in different atomic or molecular orbitals. Read on to understand abbreviated electron configuration, shells, subshell, and how to find electron configuration of an atom or element. This valence electron calculator displays the abbreviated configuration and the atomic number of each element. An online condensed electron configuration calculator helps you to determine the electron configuration of any element.


 0 kommentar(er)
0 kommentar(er)
